Last week, I submitted my third and final attempt at the NSF CAREER proposal. As with my prior two attempts, I pledged to publish the proposal openly, and I'll stick to that pledge. Despite my sleep deprivation, if I had been satisfied and proud of the proposal, I would have taken the time to post the proposal right away last week. The truth is, though, that I am demoralized and embarrassed by the proposal--all due to my own shortcomings. Well, I painted myself into a corner by promising to publish the proposal openly, so I can't hide it permanently. But as a compromise with myself, I am allowing myself to publish only the Specific Aims section now, which I am reasonably happy with. A copy can be found on slideshare: CAREER: Open-science studies of effects of water isotope and osmotic stress on biomolecular interactions. I will publish the disastrous remainder of the proposal once I have the stomach to do so in the next few weeks (before it goes out for review).
I remain happy with the Specific Aims, which many successful grant writers argue is the most important section of the proposal. What I like most is that I decided to elevate "open science" to be one of the three specific aims. I also love the science I am proposing and would love to have my students focus on this research plan over the next five years.
So why a failure? Well there are many failures, the worst and most embarrassing is that I completely ran out of time and did not submit even close to a decent final product. I guess those of you who can't avert your eyes from the worst train wreck will see this when I post the full version in a few weeks--and I'm not kidding. As I mentioned above, this is all my fault. I made a huge mistake and attempted to completely rewrite the proposal instead of tweaking the 2009 version. The 2009 proposal was declined. The reviewers were very thoughtful and liked the research plan and loved the "broader impacts" of the open science, as I described in another post. They just did not believe I could carry out the research as we did not have enough preliminary data. I think I would have had more than a 50% chance of getting it funded this time, if we'd generated a lot of preliminary data supporting the proposal over the past two years. It would have been relatively easy to revise the proposal and assuage the reviewers' concerns. However, we failed at generating much of this preliminary data. We did get some data, but not enough, and have been stymied in large part due to lack of funding. My lab was very lucky to receive funding for an unrelated project from DTRA, and thus we spent most of our resources (time and money) on that project (kinesin) as opposed to generating preliminary data for the DNA unzipping work.
I could have added in a lot of new data (and "spin") and I think the tweaked CAREER proposal would have had a shot at getting funded. I also think it would have been reasonable for NSF to fund it, because given the amount of funding ($150K / year for 5 years), we would have received the necessary resources to pursue the research. So why didn't I go this route? I wrestled with the decision for many weeks with no path obvious to me. I ultimately went with my gut and decided to scrap most of the proposed DNA unzipping research and to instead rewrite the proposal to focus on the solvent effects of water on kinesin and protein-DNA interactions. There were many pros and cons I weighed, consciously and sub-consciously, but I'd say the prominent reasons for rewriting were the following: (a) I'm worn-down by years of pursuing the DNA unzipping project without the proper financial or human resources, (b) I'm fascinated by the question of whether deuterium is essential for some cellular processes (c) I don't have the ability or desire to manage a lab that is pursuing two large and separate research projects and (d) our funded project--studying the molecular motor kinesin--is going very well, is exciting, and, frankly, seems much more doable since we've had two years of sufficient funding for it. While the kinesin grant saved my lab and allowed the graduate students to remain in our lab, it did have a side effect of diverting most of our resources from the project we had been pursuing to map native chromatin by DNA unzipping.
In retrospect, I think I made a good decision, but I waited much too long to decide, a huge mistake. I spent as much time as I could on the rewriting, but just flat-out failed. Big time. So badly I still don't have the stomach to talk about it. Because of the deadline and the point I am in my career (tenure decision this academic year), delaying submission was not an option. So, I submitted a version that is very poor in many areas. I feel bad about wasting the time of reviewers, including those from 2009 who obviously spent a lot of time coaching me on how to improve the proposal. I hope, maybe, that some of my ideas despite being poorly written will still be enjoyable to the reviewers. What I feel worst about is that I really like the research proposed in the Specific Aims. I think it's the research path I should pursue and the students would succeed and make a big impact. And I totally squandered the opportunity to have it funded by the NSF CAREER program. So, even a week later, I remain embarrassed, disappointed, disgusted, etc.
I suppose there are bright sides. As we all know, research funding rates are very low anyway--so even a perfectly-written proposal would have been lucky to have been funded. And, if I don't piss off too many reviewers, I can still pursue funding via the many other funding avenues that I need to pursue anyway. And if I don't get the funding, I think the ideas are still worth pursuing. So, by openly publishing the entire mess, maybe another group of researchers will be encouraged to pursue the open questions with their own resources.
SJK August 18, 2011: As promised, I have posted the full Project Description. Here is a link on SlideShare: http://www.slideshare.net/skoch3/2011-nsf-careersteve-koch-full-project-description
Monday, August 1, 2011
Saturday, September 4, 2010
Hey Gilbert Lewis: Has life evolved a use for deuterium? Or does it just tolerate it?
But that's not what I want to talk about tonight. Tonight, I want to ask the question: Have life forms adapted a use for deuterium? Or is it merely tolerated? To talk about this, I will go back to really fun 1933 letter to JACS by Gilbert N. Lewis1. I found this paper because of our work with D2O in the lab and my quick realization that my initial assumptions about D2O were way off. I had assumed that D2O was pretty much like regular water, just a bit denser. I completely missed the point that D (deuterium: one proton, one neutron) is chemically very different from H (hydrogen or protium, one proton, zero neutrons). This is because the reduced mass of an H-X bond is substantially different from a D-X bond, and thus the binding energy is substantially different. (X refers to some other atom such as oxygen or carbon.) It turns out that the chemistry of D is so much different from H that relatively pure D2O is toxic to eukaryotic lifeforms! D2O also has many other amazing effects on life forms and biomolecules. For example, it stabilizes microtubules2, which is a major reason it is toxic3. It also increases the thermostability of proteins4 or even whole fruit flies5. Last fall, I summarized some of the things I learned about D2O in a group meeting.
So, while I was naive in 2009 (and likely continuing 2010->), Gilbert Lewis was amazingly prescient in 1933. In his letter to JACS, he says, "Sir: Even before I had succeeded in concentrating the isotope of hydrogen, I predicted that H2H2O would not support life and would be lethal to higher organisms." Don't you wish research letters today began so strikingly??? What a breath of fresh air(rogance)! The letter goes on to describe a beautifully simple experiment that he was able to carry out to demonstrate the toxicity of D2O to tobacco seeds. He used tobacco seeds because they are tiny and he only had a small amount of D2O (that he purified himself). He showed that 6 tobacco seeds in regular water sprouted nicely over the course of two weeks. Whereas 6 tobacco seeds in reasonably pure D2O did not sprout at all. Tobacco seeds in 50/50 D2O / H2O (which would be 50% H1H2O) sprouted, but slowly. At the end of the letter, Lewis says, "I have long desired to determine the proportions of isotopes in living matter, in order to see whether the extraordinary selective power of living organisms, which is exemplified by their behavior toward optical isomers, might lead to a segregation of isotopes in some of the substances which are necessary to growth. The marked biochemical differences between the two isotopes of hydrogen lends a further incentive to this search." It's not exactly clear, but I have to guess that Lewis was wondering whether living organisms would use D and H differently, and whether he could detect this via deviations from the ratio of D to H in (standard mean) ocean water.
To me, this is a fascinating question. Do cells use D for specialized purposes? If not, do they use pumps, etc. to increase the concentration of H inside cells and reduce the toxic effects of D? When I mention this to most scientists, it seems to set off their "crackpot" sensors, which is understandable. I mentioned this to Steven Benner at a DTRA program review a few weeks ago and his crackpot sensor initially was triggered. He said, "Deuterium is only 1 hundredth of a percent [of the total hydrogens in regular water]." I said, "But that's 17 millimolar!" He said, "OK, well you changed the units on me..." But then he changed his tune a bit and I think he considered it plausible.
0.03% does sound trivial. But the way I look at it, biology has somehow evolved to make use of different divalent cations in much lower concentration, such as magnesium, zinc, calcium, etc. And it can distinguish between potassium (K+) and sodium (Na+). How much more different are K+ and Na+ from each other, compared to the difference between D and H? I actually don't know, but my intuition would say it's in the same ballpark. I find it remarkable that regular water has 17 millimolar of deuterium "contamination" in it, and up until 2009 it never occured to me that it could matter! As an example, if you're studying a proton pump at the single-molecule level, 1 out of six thousand events may be artificially slowed because D is in there instead of H. This could make a difference, especially if studying events like pausing. (There is a recent paper by Yuan and Berg that studies isotope effects in the bacterial rotary motor that I have not yet read carefully6.)
So, at some level it seems important to remember that there's a lot of deuterium in regular "pure" water. But more interesting to me is whether life has adapted uses for this deuterium. I think if Lewis had had easy access to deuterium-depleted water, he would have investigated this right away. But as far as I know, deuterium-depleted water didn't become readily available until many decades later (I could be wrong on this). I think Lewis' tobacco seed experiments are the perfect place to start studying this effect. My hypothesis is: tobacco seeds in deuterium-depleted water (<1 part per million D; Aldrich product no. 195294) will sprout more slowly than in "regular" water with approximately 150 parts per million D. To test this, we only need:
- Tobacco seeds (cheap, right?)
- Deuterium-depleted water $25
- 500 microliter microfuge tubes, a camera (maybe microscope?), and some time
I'm obviously not the first person to think of this. I'm sure Lewis thought of it, but didn't have the resources. D-depleted water is cheap nowadays, probably because of its use in NMR. A google search for "deuterium-depleted water" is ruined by a huge amount of links to products about the use of D-depleted water for curing all kinds of cancer. This is unfortunate. Digging around, I was able to find a couple peer-reviewed papers investigating the effects of deuterium depletion on life. The first I found is by Somlyai et all in FEBS 19937. They claim to prove that "naturally-occurring deuterium is essential for normal cell growth." This paper has been cited 10 times, and of those 10, I think only two investigate deuterium-depletion effects. Those two are by the same research group. Their results would be striking, but it is not convincing for at least two reasons. First, their level of depletion was not substantial: 150 ppm to 30-40 ppm (compared to <1 ppm which we can achieve now). Secondly, they investigate the effects on human cell lines. As far as I know, human cell lines are finicky...much, much more finicky than tobacco seeds. I think it was a mistake to jump right into cell lines.
The results of Somlyai et al. may be true, and if so would be exciting. Not, in my opinion, as an immediate cure for cancer, but rather as a fascinating new area of cell biology to study. I think a quick extension of Lewis' beautiful tobacco seed experiments is a great first step. If we don't see an effect? Try again! Maybe try mustard seed too. Steven Benner, indicated the next step (paraphrasing from fuzzy memory): grow yeast or E. coli in D-depleted water. Check for which genes are mutated. Those genes are candidates for encoding proteins that utilize deuterium for the benefit of the cell. Or proteins that sequester D, I suppose.
So, what do you think? Should we try this experiment? I think so, and I'll write up a proposed protocol in my OpenWetWare notebook soon (I hope).
PS: In addition to the short letter by Lewis I talked about above, I highly recommend reading a longer letter to Science in 19348. In his first paragraph he says, "Several months ago the experiments were interrupted, and since there may be no immediate opportunity of resuming them it seems best to publish the somewhat sporadic results so far obtained." Don't you wish you could publish your own sporadic results in Science? It's a very interesting paper where he describes further tobacco seed experiments as well as microorganisms, flatworms, and mice.
References (Send me a note if you'd like the PDF for the ref. 1)
1. Gilbert N. Lewis (1933). THE BIOCHEMISTRY OF WATER CONTAINING HYDROGEN ISOTOPE Journal of the American Chemical Society, 55 (8), 3503-3504 DOI: 10.1021/ja01335a509
2. Panda, D., Chakrabarti, G., Hudson, J., Pigg, K., Miller, H. P., Wilson, L., et al. (2000). Suppression of microtubule dynamic instability and treadmilling by deuterium oxide. Biochemistry, 39(17), 5075-81. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10819973.
3. I think there is a study from 1935 that looks at cells in D2O and sees larger spindle apparatus in mitotic cells, but I can't find the reference now.
4.
FriendFeed thread:
Wednesday, April 1, 2009
Should new investigators apply for NIH Challenge Grants (RC1)?
I am looking more into the NIH Challenge Grant (RC1) opportunities that are part of the US stimulus bill. The specific question in my mind right now is: "Does it make sense for new investigators (like me) to apply for RC1 grants?" The reasons I ask are (a) because winning an RC1 removes the "new investigator" status from a PI, and (b) there is no "new investigator" preference in RC1 reviews.
The NIH has a specific definition for "new investigator", which is any investigator who has not been PI on any PHS-supported project other than a "small" one, such as a K-award or R-21 grant. New investigators get many benefits in review of R01 grants, including:
Thus, if I were to win a two-year RC1, I would no longer be considered a "new investigator" for future R01 applications. This is a serious issue to consider. Two years of funding would be great, but five years of funding would be much better. Furthermore, this rule, combined with other language in the RFA makes me wonder whether new investigators will be frowned upon overall in the review process?
Does anyone else have any thoughts on this issue? I am heavily leaning towards not applying for RC1. But I also know that I'm biased by the fact that I just submitted a couple grant applications (to other agencies) and the thought of doing another one in the next three weeks is really not appealing!
SJK Note added 8:06 PM: Another negative is that there are no resubmissions of RC1s, since it is a one-time program.
SJK Note 4/2/09: Friendfeed comments.

The NIH has a specific definition for "new investigator", which is any investigator who has not been PI on any PHS-supported project other than a "small" one, such as a K-award or R-21 grant. New investigators get many benefits in review of R01 grants, including:
- Instructions to the study section to go easy on the new investigators (again, see the link from the Center for Scientific Review).
- Center-specific practices to increase paylines and grant duration for new investigators. For example, the NCI in the past extended the payline from the 11th percentile to the 16th percentile. The NHGRI was not as specific, but they also increased the payline and also strive to support new investigators for 5 years.
New PIs and Early Stage Investigators (ESIs) are invited to apply for Recovery Act Challenge Grants in Health and Science Research. Because the awards made under this program are substantial competing NIH research grants, recipients will not be considered New PIs or ESIs when they apply for NIH research grants in the future.
Thus, if I were to win a two-year RC1, I would no longer be considered a "new investigator" for future R01 applications. This is a serious issue to consider. Two years of funding would be great, but five years of funding would be much better. Furthermore, this rule, combined with other language in the RFA makes me wonder whether new investigators will be frowned upon overall in the review process?
Does anyone else have any thoughts on this issue? I am heavily leaning towards not applying for RC1. But I also know that I'm biased by the fact that I just submitted a couple grant applications (to other agencies) and the thought of doing another one in the next three weeks is really not appealing!
SJK Note added 8:06 PM: Another negative is that there are no resubmissions of RC1s, since it is a one-time program.
SJK Note 4/2/09: Friendfeed comments.

Sunday, March 15, 2009
Tapping a new quadrant in the single-molecule research world: single-molecule genetics
Anthony Salvagno, a grad student in our lab recently described his encounter with the fear of scooping on our lab blog. In one of my responses to him in the comments, I state how I really don't think we're competing with the Wang lab, or any other lab, as far as I know at this moment. We are, of course, in competition with other labs, in terms of struggle to obtain a share of limited research funding. But I don't want our lab to get mired in direct competition and "race to publish first" that sometimes occurs. I'm not so worried about this, though, because I think we have a whole slew of important and novel ideas that we can pursue--much more than we have the manpower for. As Michael Nielson pointed out to me in the comments to his blog post, most scientists are in this boat: they have far more good research ideas than they can pursue. This is what makes intentional scooping a rare event in my mind -- it is only carried out by those paranoid, non-creative PIs whose fear of failure forces them to steal other's ideas and possibly crush younger scientists along the way. I think as Open Science (aka Science 2.0) takes over in the next decades, episodes of intentional scooping will become much, much easier to punish, due to the public track record of research progress and grant proposals available for all to see.
In this post, I want to elaborate on why I think our ideas are important but unique, and therefore not in direct competition with most of the leading labs in the single-molecule manipulation world today. The table below illustrates why I think we can lead a new era of experiments in an under-tapped area of single-molecule analysis--"single-molecule genetics." I am using a 2x2 matrix to analyze the research space. This is a technique I learned during the end of my graduate career when I interviewed and almost landed a job with Boston Consulting Group (BCG, a company I highly respected). The top row represents ensemble assays where the properties of many molecules are averaged together. The bottom row represents the single-molecule analysis research world. The left column represents experiments where the system being studied has been reconstituted from purified components. The right column represents experiments where the system being studied was intact, with only a few specific genes having been mutated or knocked-down*.
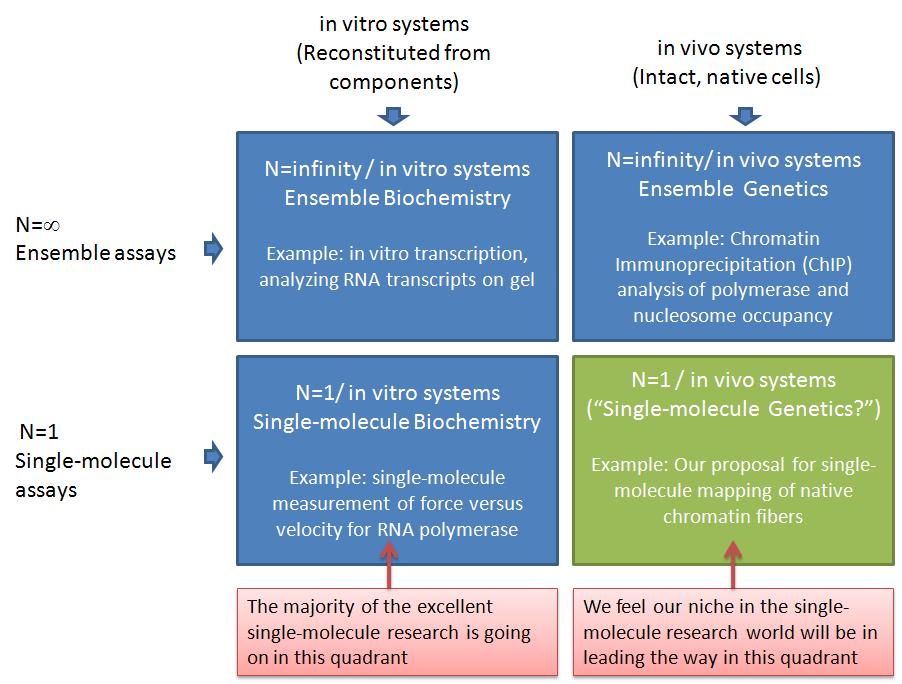
The top left quadrant represents what people refer to as biochemistry research, whereas the top right quadrant would be genetics**. The vast majority of the important discoveries in biology in the past half century would be in the top row (ensemble assays). Single-molecule assays in the bottom row have been very valuable, but are only now becoming more widely available. In my opinion, the vast majority of single-molecule assays have been in in the bottom left quadrant, which I am calling "single-molecule biochemistry" for convenience. For example, the Steve Block lab has made a number of breakthroughs in this quadrant. The Block Lab is a good example, because the research and papers produced by the students and postdocs in this lab over the past couple decades are of the highest quality and have played a key role in defining the single-molecule field. One of their areas of study is RNA Polymerase, where they have applied forces to recombinant E. coli RNA Polymerase during elongation in all sorts of manners: opposing elongation, assisting elongation, pulling on the RNA transcript, etc. Another main area of the block lab is the molecular motor kinesin. They have used optical tweezers to make many important discoveries about the mechanochemistry of this amazing enzyme. From these two areas combined (which still account for only part of the Block Lab research), they have something like 10,000 Science, Nature, and Cell papers. And as far as I know, all of that research has been done using dozens of different recombinant RNA Polymerase and kinesin heavy chain (KHC) motor proteins. So, as far as my matrix goes, they have made a huge impact on the bottom left quadrant -- "single molecule biochemsitry."
As I've said, I think the bottom right quadrant has so far been under-utilized. I am calling this quadrant "single-molecule genetics," to highlight what I see as the untapped power of combining proven single-molecule analyses with existing genetics techniques. When I say "untapped," I don't mean to imply that there aren't existing studies in this quadrant. I only mean that most of the research (and money) from single-molecule manipulation groups I am familiar with has been going into the SM biochemistry quadrant. This includes groups such as those of Steve Block, Carlos Bustamante, Evan Evans, Mark Williams, Jeff Gelles, Vincent Croquette, David Bensimon, Steve Kowalczykowski, Julio Fernandez, Matthias Rief, Herman Gaub, Sanford Leuba, Rich Superfine, Michelle Wang, and many others. I believe the labs I just mentioned perform research primarily in the "single-molecule biochemistry" quadrant. There are many examples of outstanding single-molecule analyses in the bottom-right quadrant. One of my recent favorites is from Osheim, Sikes, and Beyer where they used electron microscopy (EM) visualization of chromatin fibers extracted from Drosophila to study Pol II termination at the single-molecule level. "DNA fiber analysis" is another great example of research in this quadrant. The biomembrane force probe that Evan Evans' lab uses also lends itself to research that I would put in this quadrant. For example, the work of Heinrich, Leung, and Evans, studied ligand-receptor interactions on living human neutrophils. Another important example is from Cui and Bustamante, who studied the mechanical properties of individual native chromatin fibers from chicken erythrocytes.
The Cui and Bustamante work is closest to the killer application we are pursuing. We are working to use single-molecule DNA unzipping to map the positions of nucleosomes and polymerases on specific native chromatin fibers. This will be different from the Cui and Bustamante work in a number of ways. First, we expect to be able to map the positions of nucleosomes and polymerases with close to basepair accuracy. Second, we will analyze positions on site-specific chromatin fibers. Thus, we will know which gene it is, where the promoters and terminators are, etc. You can read more about our ideas in a recent minigrant proposal which I posted on Scribd. (This was funded by the way!) Our single-molecule research will complement the ensemble studies currently used -- commonly Chromatin Immunoprecipitation (ChIP). Because we will analyze chromatin extracted from living yeast cells (and higher organisms in the future), we will be able to study chromatin remodeling as genes are turned on or off and in any mutant strains we'd like. This is the same genetics as is carried out in the ensemble assays (top right quadrant), and in fact, our collaborators (Mary Ann Osley lab) currently do much research in this quadrant. We think our single-molecule method will be particularly good for addressing many open questions related to chromatin remodeling during transcription. For example, the Osley lab recently showed that a yeast double mutant deficient in FACT and H2b-ubiquitylation has an interesting phenotype that seems to have some kind of misassembled chromatin. Deciphering this chromatin structure is difficult with ensemble assays, and single-molecule analysis can shed a lot of light on this question. For example, if the chromatin is being assembled with histone tetramers instead of octamers, that should be clearly visible in the single-molecule unzipping signals.
By pursuing research in the "single-molecule genetics" or bottom-right quadrant, I think we are poised for making important contributions that complement the other quadrants. I just illustrated how we can closely complement ensemble genetics experiments (ChIP). Further, we complement single-molecule biochemstry experiments in the bottom-left and ensemble biochemistry in the top-left. As far as Pol II experiments go I don't believe any single-molecule force v. velocity transcription assays have been carried out yet. The work of Shundrovsky, Hall, and others in the Wang lab in terms of unzipping reconstituted mononucleosomes is in the bottom-left quadrant, and it certainly complements our work, because we're relying on their results to know what to look for when unzipping native chromatin fibers. And we'll do some of our own work in this quadrant in terms of unzipping Pol II in vitro transcription complexes for a similar purpose. In contrast to nucleosomes, we expect the Pol II unzipping signature to look distinct for unzipping from upstream versus downstream. If so, this will allow us to determine the sense versus antisense orientation of polymerases on native chromatin fibers, giving us single-molecule insight into a very exciting area of eukaryotic transcription. (See, for example, a recent antisense transcription paper from Core, Waterfall, and Lis.)
OK, hopefully I succeeded at least a bit in describing how I think we can lead a new area of single-molecule research: single-molecule genetics. I'd be curious in hearing whether this "2x2 matrix" helps you at all in looking at the research space. I find it very useful -- but on the other hand, I also really enjoyed a business course I took, and I thought the interview process for BCG was really fun. So, I may be a little different. If you do find it useful, I have some other 2x2's I can talk about. For example, Single-molecule/Ensemble versus With Force / Without Force.
*Footnote on "in vivo" terminology.
I am using "in vivo" to designate that the biology occurred in the context of a native cell, even if the analysis of the molecules was carried out with an in vitro assay. This would be true, in Chromatin Immunoprecipitation studies of chromatin remodeling during transcription, for example. The transcription is carried out in the nucleus, then the cells are fixed with formaldehyde and analysis is carried out in vitro. I am consdering this an "in vivo" experiment, in contrast to studies where the transription has been carried out in vitro -- such as in the amazing reconstituted systems of the Reinberg lab and others.
Certainly the holy grail of "in vivo" would be to know the 3-D position and chemical nature of every molecule in real-time while the cell is still living. And many single-molecule researchers are making big strides towards this goal by visualizing and tracking individual molecules inside living cells. However, my focus here is more on the arena of single-molecule manipulation.
**Footnote on doo doo
I may not be getting the of biochemistry and genetics exactly correct, and I do know that there is some ongoing rivalry between these two fields. John Lis, one of my science heroes who taught a molecular biology course I took told us two quotations. I wish I could remember who to attribute these to:
Famous geneticist: "Genetics without biochemistry is doo-doo."
Extremely measured response from famous biochemist: "Biochemistry without genetics is an exercise in frustration."
SJK Note 1: I don't even know enough to know whether I've remembered these correctly. Possibly interchange genetics<-->biochemistry, etc. but you get the point: they are complementary research fields and there is a rivalry.
SJK Note 2: If you know a source for these quotes, please post a comment! I couldn't find it on Google. Also see some interesting related discussion on friendfeed.

In this post, I want to elaborate on why I think our ideas are important but unique, and therefore not in direct competition with most of the leading labs in the single-molecule manipulation world today. The table below illustrates why I think we can lead a new era of experiments in an under-tapped area of single-molecule analysis--"single-molecule genetics." I am using a 2x2 matrix to analyze the research space. This is a technique I learned during the end of my graduate career when I interviewed and almost landed a job with Boston Consulting Group (BCG, a company I highly respected). The top row represents ensemble assays where the properties of many molecules are averaged together. The bottom row represents the single-molecule analysis research world. The left column represents experiments where the system being studied has been reconstituted from purified components. The right column represents experiments where the system being studied was intact, with only a few specific genes having been mutated or knocked-down*.

The top left quadrant represents what people refer to as biochemistry research, whereas the top right quadrant would be genetics**. The vast majority of the important discoveries in biology in the past half century would be in the top row (ensemble assays). Single-molecule assays in the bottom row have been very valuable, but are only now becoming more widely available. In my opinion, the vast majority of single-molecule assays have been in in the bottom left quadrant, which I am calling "single-molecule biochemistry" for convenience. For example, the Steve Block lab has made a number of breakthroughs in this quadrant. The Block Lab is a good example, because the research and papers produced by the students and postdocs in this lab over the past couple decades are of the highest quality and have played a key role in defining the single-molecule field. One of their areas of study is RNA Polymerase, where they have applied forces to recombinant E. coli RNA Polymerase during elongation in all sorts of manners: opposing elongation, assisting elongation, pulling on the RNA transcript, etc. Another main area of the block lab is the molecular motor kinesin. They have used optical tweezers to make many important discoveries about the mechanochemistry of this amazing enzyme. From these two areas combined (which still account for only part of the Block Lab research), they have something like 10,000 Science, Nature, and Cell papers. And as far as I know, all of that research has been done using dozens of different recombinant RNA Polymerase and kinesin heavy chain (KHC) motor proteins. So, as far as my matrix goes, they have made a huge impact on the bottom left quadrant -- "single molecule biochemsitry."
As I've said, I think the bottom right quadrant has so far been under-utilized. I am calling this quadrant "single-molecule genetics," to highlight what I see as the untapped power of combining proven single-molecule analyses with existing genetics techniques. When I say "untapped," I don't mean to imply that there aren't existing studies in this quadrant. I only mean that most of the research (and money) from single-molecule manipulation groups I am familiar with has been going into the SM biochemistry quadrant. This includes groups such as those of Steve Block, Carlos Bustamante, Evan Evans, Mark Williams, Jeff Gelles, Vincent Croquette, David Bensimon, Steve Kowalczykowski, Julio Fernandez, Matthias Rief, Herman Gaub, Sanford Leuba, Rich Superfine, Michelle Wang, and many others. I believe the labs I just mentioned perform research primarily in the "single-molecule biochemistry" quadrant. There are many examples of outstanding single-molecule analyses in the bottom-right quadrant. One of my recent favorites is from Osheim, Sikes, and Beyer where they used electron microscopy (EM) visualization of chromatin fibers extracted from Drosophila to study Pol II termination at the single-molecule level. "DNA fiber analysis" is another great example of research in this quadrant. The biomembrane force probe that Evan Evans' lab uses also lends itself to research that I would put in this quadrant. For example, the work of Heinrich, Leung, and Evans, studied ligand-receptor interactions on living human neutrophils. Another important example is from Cui and Bustamante, who studied the mechanical properties of individual native chromatin fibers from chicken erythrocytes.
The Cui and Bustamante work is closest to the killer application we are pursuing. We are working to use single-molecule DNA unzipping to map the positions of nucleosomes and polymerases on specific native chromatin fibers. This will be different from the Cui and Bustamante work in a number of ways. First, we expect to be able to map the positions of nucleosomes and polymerases with close to basepair accuracy. Second, we will analyze positions on site-specific chromatin fibers. Thus, we will know which gene it is, where the promoters and terminators are, etc. You can read more about our ideas in a recent minigrant proposal which I posted on Scribd. (This was funded by the way!) Our single-molecule research will complement the ensemble studies currently used -- commonly Chromatin Immunoprecipitation (ChIP). Because we will analyze chromatin extracted from living yeast cells (and higher organisms in the future), we will be able to study chromatin remodeling as genes are turned on or off and in any mutant strains we'd like. This is the same genetics as is carried out in the ensemble assays (top right quadrant), and in fact, our collaborators (Mary Ann Osley lab) currently do much research in this quadrant. We think our single-molecule method will be particularly good for addressing many open questions related to chromatin remodeling during transcription. For example, the Osley lab recently showed that a yeast double mutant deficient in FACT and H2b-ubiquitylation has an interesting phenotype that seems to have some kind of misassembled chromatin. Deciphering this chromatin structure is difficult with ensemble assays, and single-molecule analysis can shed a lot of light on this question. For example, if the chromatin is being assembled with histone tetramers instead of octamers, that should be clearly visible in the single-molecule unzipping signals.
By pursuing research in the "single-molecule genetics" or bottom-right quadrant, I think we are poised for making important contributions that complement the other quadrants. I just illustrated how we can closely complement ensemble genetics experiments (ChIP). Further, we complement single-molecule biochemstry experiments in the bottom-left and ensemble biochemistry in the top-left. As far as Pol II experiments go I don't believe any single-molecule force v. velocity transcription assays have been carried out yet. The work of Shundrovsky, Hall, and others in the Wang lab in terms of unzipping reconstituted mononucleosomes is in the bottom-left quadrant, and it certainly complements our work, because we're relying on their results to know what to look for when unzipping native chromatin fibers. And we'll do some of our own work in this quadrant in terms of unzipping Pol II in vitro transcription complexes for a similar purpose. In contrast to nucleosomes, we expect the Pol II unzipping signature to look distinct for unzipping from upstream versus downstream. If so, this will allow us to determine the sense versus antisense orientation of polymerases on native chromatin fibers, giving us single-molecule insight into a very exciting area of eukaryotic transcription. (See, for example, a recent antisense transcription paper from Core, Waterfall, and Lis.)
OK, hopefully I succeeded at least a bit in describing how I think we can lead a new area of single-molecule research: single-molecule genetics. I'd be curious in hearing whether this "2x2 matrix" helps you at all in looking at the research space. I find it very useful -- but on the other hand, I also really enjoyed a business course I took, and I thought the interview process for BCG was really fun. So, I may be a little different. If you do find it useful, I have some other 2x2's I can talk about. For example, Single-molecule/Ensemble versus With Force / Without Force.
*Footnote on "in vivo" terminology.
I am using "in vivo" to designate that the biology occurred in the context of a native cell, even if the analysis of the molecules was carried out with an in vitro assay. This would be true, in Chromatin Immunoprecipitation studies of chromatin remodeling during transcription, for example. The transcription is carried out in the nucleus, then the cells are fixed with formaldehyde and analysis is carried out in vitro. I am consdering this an "in vivo" experiment, in contrast to studies where the transription has been carried out in vitro -- such as in the amazing reconstituted systems of the Reinberg lab and others.
Certainly the holy grail of "in vivo" would be to know the 3-D position and chemical nature of every molecule in real-time while the cell is still living. And many single-molecule researchers are making big strides towards this goal by visualizing and tracking individual molecules inside living cells. However, my focus here is more on the arena of single-molecule manipulation.
**Footnote on doo doo
I may not be getting the of biochemistry and genetics exactly correct, and I do know that there is some ongoing rivalry between these two fields. John Lis, one of my science heroes who taught a molecular biology course I took told us two quotations. I wish I could remember who to attribute these to:
Famous geneticist: "Genetics without biochemistry is doo-doo."
Extremely measured response from famous biochemist: "Biochemistry without genetics is an exercise in frustration."
SJK Note 1: I don't even know enough to know whether I've remembered these correctly. Possibly interchange genetics<-->biochemistry, etc. but you get the point: they are complementary research fields and there is a rivalry.
SJK Note 2: If you know a source for these quotes, please post a comment! I couldn't find it on Google. Also see some interesting related discussion on friendfeed.

Saturday, January 24, 2009
American Cancer Society IRG proposal submitted!
We submitted our proposal for the internal American Cancer Society (ACS) Institutional Research Grant (IRG) yesterday. I've uploaded it to Scribd, in case you'd like to look. I am really happy with it! And I am really proud of the students in my lab for both their help on the proposal, and for their accomplishments in getting our new lab to point we are now. I feel like this year is going to be a whole lot of really great science and many accomplishments from them. I'd also really like to thank Mary Ann Osley and Kelly Trujillo, our collaborators at UNM, for help in writing this and developing the ideas. These ideas were developed with the help of Karen Adelman (NIH NIEHS) also. Thank you everyone!
Wednesday, January 21, 2009
Specific Aims draft for upcoming miniproposal
I've uploaded a draft to Scribd of the specific aims section for a miniproposal I have due on Friday. The proposal is for $30K from an American Cancer Society (ACS) Institutional Research Grant (IRG) for one year of research. It is a renewal of the previous IRG we received which provided us with $22,500 for one year of research and for which we have been extremely grateful. While that kind of funding is much smaller than the NIH R01 funding we're striving for, it makes a big impact on our lab. I think any lab would appreciate that kind of funding, but it's particularly valuable to us in this start-up phase.
The proposal is due Friday (Jan. 23). To me, these specific aims seem very clear and I like the way they're written...so I think it's almost finished. But if you do happen to read it before Friday morning (or even after), I'd love to have your comments!
And, I should mention that this proposal is 7 pages in "NIH-style." That means it has sections of:
PS: Thank you to Anthony, Andy, and Larry for help in writing this, and for all the help from our collaborators, Mary Ann, Kelly, and Karen!
The proposal is due Friday (Jan. 23). To me, these specific aims seem very clear and I like the way they're written...so I think it's almost finished. But if you do happen to read it before Friday morning (or even after), I'd love to have your comments!
And, I should mention that this proposal is 7 pages in "NIH-style." That means it has sections of:
- Specific Aims
- Background and Siificance
- Preliminary Studies
- Research Plan
PS: Thank you to Anthony, Andy, and Larry for help in writing this, and for all the help from our collaborators, Mary Ann, Kelly, and Karen!
Monday, January 19, 2009
Writing our first paper--Shotgun DNA Mapping
Today, our lab created a sort-of-complete draft of the first paper to come out of our lab. I wrote briefly about it on our kochlab research blog and also posted our draft on scribd. I don't want to re-blog everything here so I'll just say that we're really excited about the paper and if you happen to click on those links above, I (we) would love to hear your opinions.
I'm really proud of the lab members, and especially Larry, who did most of the programming and produced the results that are in this paper. He produced much of this during two amazingly productive months last year (say August/September-ish) when he was seemingly-happily working 7 days a week with many 10+ hour days. If you happened to read my previous blog about talents in the lab on my science blog, you may have just realized that that kind of dedication and productivity is indicative of some kind of underlying talent that Larry has, and I wholeheartedly agree. Larry and I recognized this during our recent "talents meeting," and we're wanting to analyze that time period as a way of more clearly seeing what these strong talents are.
In contrast to the research productivity, we've been wanting to draft this paper for several months now, and up until today it was painful for both of us. I definitely committed some management sins (I'm avoiding a euphemism we use in this situation), but I think he's forgiven me by now. Last week, we fully consciously realized that the writing process we'd been attempting was failing and so we decided to try something new. The process we had been trying was to collaborate on our private wiki by having Larry draft sections and I and others commenting on his work and making suggestions. There are probably many reasons this failed and I know many of them are due to non-talents I have with writing and management. The new process we tried today was to just block off a whole day in a conference room to work together towards a single outcome: to produce a document in Word that was a reasonably coherent draft of the paper that we could distribute to our friends and collaborators for advice. Anthony, Linh, Larry, and I did this today, and I think the experiment was highly successful. Certainly the draft we produced is very rough, and none of us will be surprised if >75% of it is changed as we revise and pound on it in the coming weeks. But I think we surpassed the outcome we were looking for and for sure we have achieved a very solid foothold towards our goal of actually publishing this paper in a refereed journal.
This process took us about 9 hours together, plus who knows how many hundreds of hours of discussions and prior writing attempts that we built upon. We were able to fight through several instances of writer's block today, particularly with the introduction, and I think the clear outcome we were pursuing was a huge help in pushing us through this. Many may question whether it's practical for me (or any of us) to schedule an entire day for writing a paper draft, and I hold the same question. I'm not sure whether we can or will need to do this in the future, but given the success, I think we'll try. Time like this is precious for all of us, but we received quite a valuable payout. I present my first lecture tomorrow (in about 13 hours) and I haven't made any preparation except for what I have from last year and a bunch of mostly subconscious scheming and worrying. I'll talk about this on my teaching blog, perhaps later tonight. I'm super-happy about the progress we made today, and very proud of my students ... and I also now have a really uneasy feeling in my stomach in anticipation of being unprepared for the onslaught of teaching and the grant deadlines. I should mention that the uneasiness is probably compounded by hunger, which would be the subject of another blog that I don't have.
I'm really proud of the lab members, and especially Larry, who did most of the programming and produced the results that are in this paper. He produced much of this during two amazingly productive months last year (say August/September-ish) when he was seemingly-happily working 7 days a week with many 10+ hour days. If you happened to read my previous blog about talents in the lab on my science blog, you may have just realized that that kind of dedication and productivity is indicative of some kind of underlying talent that Larry has, and I wholeheartedly agree. Larry and I recognized this during our recent "talents meeting," and we're wanting to analyze that time period as a way of more clearly seeing what these strong talents are.
In contrast to the research productivity, we've been wanting to draft this paper for several months now, and up until today it was painful for both of us. I definitely committed some management sins (I'm avoiding a euphemism we use in this situation), but I think he's forgiven me by now. Last week, we fully consciously realized that the writing process we'd been attempting was failing and so we decided to try something new. The process we had been trying was to collaborate on our private wiki by having Larry draft sections and I and others commenting on his work and making suggestions. There are probably many reasons this failed and I know many of them are due to non-talents I have with writing and management. The new process we tried today was to just block off a whole day in a conference room to work together towards a single outcome: to produce a document in Word that was a reasonably coherent draft of the paper that we could distribute to our friends and collaborators for advice. Anthony, Linh, Larry, and I did this today, and I think the experiment was highly successful. Certainly the draft we produced is very rough, and none of us will be surprised if >75% of it is changed as we revise and pound on it in the coming weeks. But I think we surpassed the outcome we were looking for and for sure we have achieved a very solid foothold towards our goal of actually publishing this paper in a refereed journal.
This process took us about 9 hours together, plus who knows how many hundreds of hours of discussions and prior writing attempts that we built upon. We were able to fight through several instances of writer's block today, particularly with the introduction, and I think the clear outcome we were pursuing was a huge help in pushing us through this. Many may question whether it's practical for me (or any of us) to schedule an entire day for writing a paper draft, and I hold the same question. I'm not sure whether we can or will need to do this in the future, but given the success, I think we'll try. Time like this is precious for all of us, but we received quite a valuable payout. I present my first lecture tomorrow (in about 13 hours) and I haven't made any preparation except for what I have from last year and a bunch of mostly subconscious scheming and worrying. I'll talk about this on my teaching blog, perhaps later tonight. I'm super-happy about the progress we made today, and very proud of my students ... and I also now have a really uneasy feeling in my stomach in anticipation of being unprepared for the onslaught of teaching and the grant deadlines. I should mention that the uneasiness is probably compounded by hunger, which would be the subject of another blog that I don't have.
Subscribe to:
Comments (Atom)



